Milestones
ACE’s Journey to Advancing Value-Based Healthcare in Singapore
On this page
Charting a Decade of Progress
Strategic Shifts: Growing ACE’s Role in Singapore’s Healthcare Landscape
From a small HTA function to a national enabler of AVBC, ACE’s evolution reflects a growing role in shaping health policy and care delivery. Over the years, we have sharpened our focus, enhanced methodological rigour, and brought greater transparency in decision-making—all to support more consistent, evidence-informed care across the health system.
2015

August
Established by MOH, ACE was set up to drive appropriate care through evaluating the clinical and cost-effectiveness of health technologies. From its inception, ACE championed evidence-based decision-making in healthcare to help ensure patients have access clinically proven, cost-effective treatments that improve health outcomes in a sustainable manner.
2016
April
The Pharmacoeconomic Evaluation and Drug Utilisation (PEDU) unit in Health Sciences Authority (HSA) supporting the MOH Drug Advisory Committee (DAC) was transferred to ACE.
May
ACE Council was formed to help guide ACE’s development and drive appropriate care and value-based healthcare.
2017
 for web 7.png)
February
Drug Evaluation Methods and Process Guide published—a key milestone that formalised ACE’s rigorous approach to Health Technology Assessment (HTA), bringing Singapore’s standards in line with international best practices and increasing transparency in evaluation and subsidy decision-making.
May
Published first tranche of drug guidances, increasing the transparency around MOH Drug Advisory Committee’s (DAC’s) subsidy decisions, in alignment with international best practices, and improving public understanding of how cost-effectiveness informs access.
July
Published first tranche of clinical guidances, “Appropriate Care Guides” (ACGs), to support specific areas of clinical practice.
2018

March
First tranche of Medical Technology guidances was published, increasing the transparency of MOH Medical Technology Advisory Committee’s (MTAC’s) subsidy decision-making for medical technologies.
May
To support the adoption of evidence-based medicine, the Appropriate Care Office (now known as the Evidence to Practice Office, ETPO) was established to empower healthcare professionals in the delivery of appropriate care by developing and facilitating adoption of national-level clinical guidances.
2019

July
Launched Horizon scanning capabilities, initially focusing on identifying emerging Cell, Tissue and Gene Therapy Products (CTGTP).
Introduced a formal framework for company submissions and cost-recovery, enabling transparent and sustainable pathways for industry engagement—developed in consultation with stakeholders and aligned with international best practices.
October
Introduced enhanced clinical guidance with the rebranded “ACE Clinical Guidances” (ACGs), adopting robust guideline development processes and methodologies aligned with international best practices.
Rolled out ACE Clinical Update Service (ACE CUES), Singapore’s national educational visiting service for healthcare professionals.
2020
July
Horizon scanning for medical technologies became a formal workstream in ACE to enable better healthcare system preparedness and resource planning.
2021
April
Established Consumer Engagement and Education (CEE) unit to involve patients and the public in ACE’s work.
September
Published the first Horizon Scanning Methods and Process Guide.
Transforming Access and Affordability
Transformative Initiatives: Making Value Work for Patients
These landmark initiatives redefined how patients access care, how treatments are funded, and how value is delivered in Singapore.
2016-2017

Piloted Value-Based Pricing (VBP) for selected health technologies, laying the groundwork for sustainable cost management and provision of cost-effective care. VBP became a core workstream in 2017.
2017
.jpg)
May
Launched the ACE website to improve access to HTAs, clinical guidances, and educational resources for healthcare professionals and the public.
2019

April
Negotiated prices with vaccine manufacturers to improve affordability and access to nationally recommended vaccines at General Practitioner (GP) clinics under the Community Health Assist Scheme (CHAS), marking a first for private GPs.
2021
January
Introduced company-led submission process for treatments, facilitating timely patient access to effective therapies.
August
Successfully signed first tranche of risk sharing agreements to manage budget certainty for high-cost cancer drugs. This enables MOH to subsidise these treatments in a sustainable manner, while supporting patient access and safeguarding healthcare resources.
2022

August
MOH introduced the Cancer Drug List (CDL) to help ensure the cost of cancer treatments and insurance premiums remain affordable over time. ACE supported its development by evaluating therapies for their evidence and value, informing listing decisions, and negotiating better prices.
Covers clinically proven and cost-effective cancer drug-indication pairs
Introduced more granular claim limits for outpatient cancer drugs
Achieved 30% price reduction for ~100 cancer drugs and >60% for some specific cancer drugs
About 90% of HSA approved cancer drug-indication pairs are listed on CDL
2023
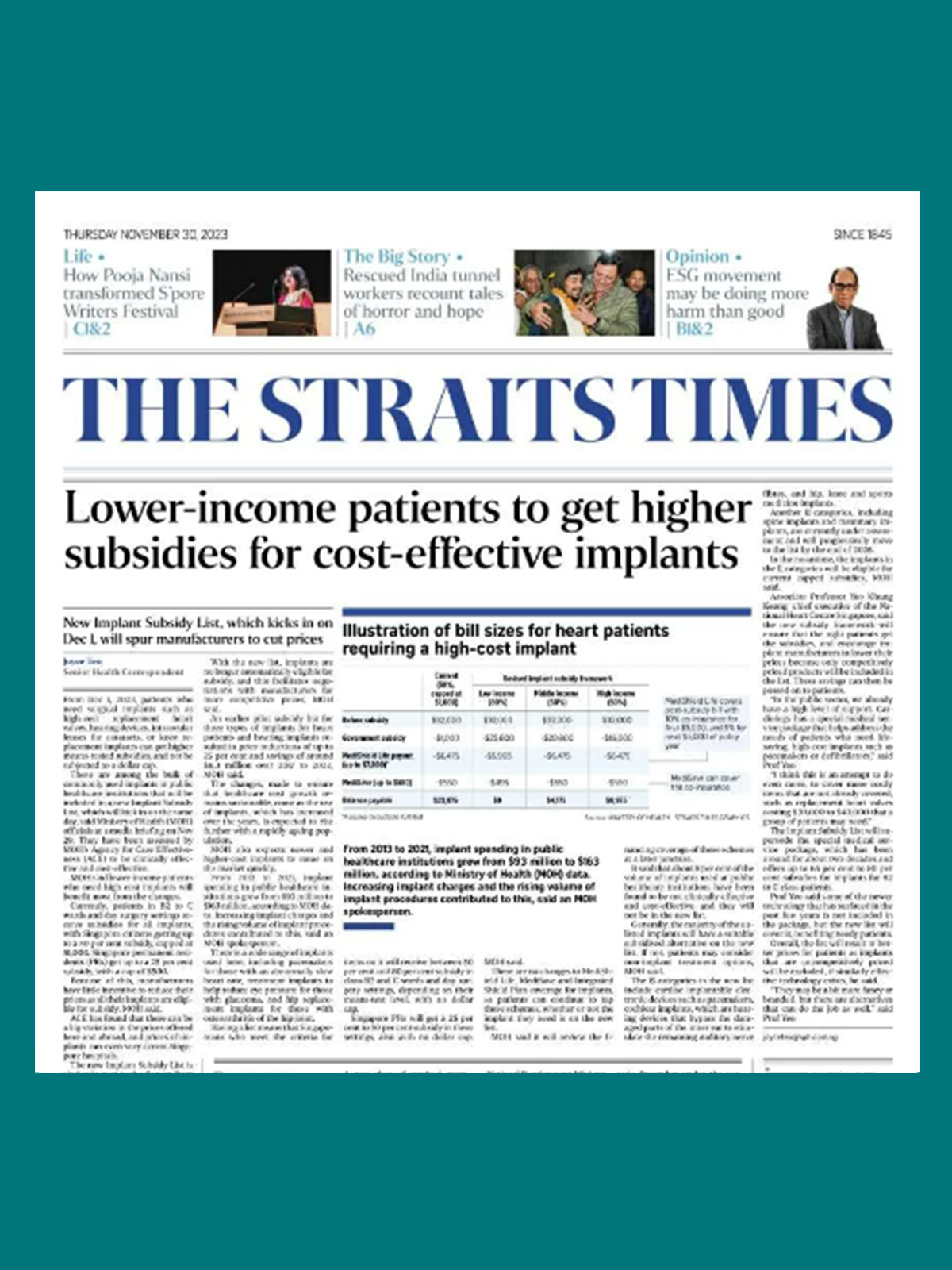
March
Introduction of the Implant Subsidy List (ISL) — Singapore’s first positive list for implants with demonstrated therapeutic and economic value. The ISL extends subsidies to encourage more consistent use of implants that improve patient outcomes and healthcare resource utilisation. Covers clinically and cost-effective implants with enhanced subsidies
Achieved 30% price reduction for commonly used implants
For implants categories that transited to ISL, >90% of implants used in public hospitals included in ISL at the time of list finalisation.
Extended negotiated Value-Based prices for whitelisted drugs to HealthierSG GPs to improve affordability in primary care.
May
Started voluntary industry notification process to provide early visibility of pipeline medical technologies to MOH for early resource planning.
December
ACE evaluated the clinical and cost-effectiveness of genetic testing for familial hypercholesterolaemia (FH). This assessment informed the design of optimal clinical workflows to maximise the value of testing in real-world practice, supporting the integration of precision medicine into routine care.
2024
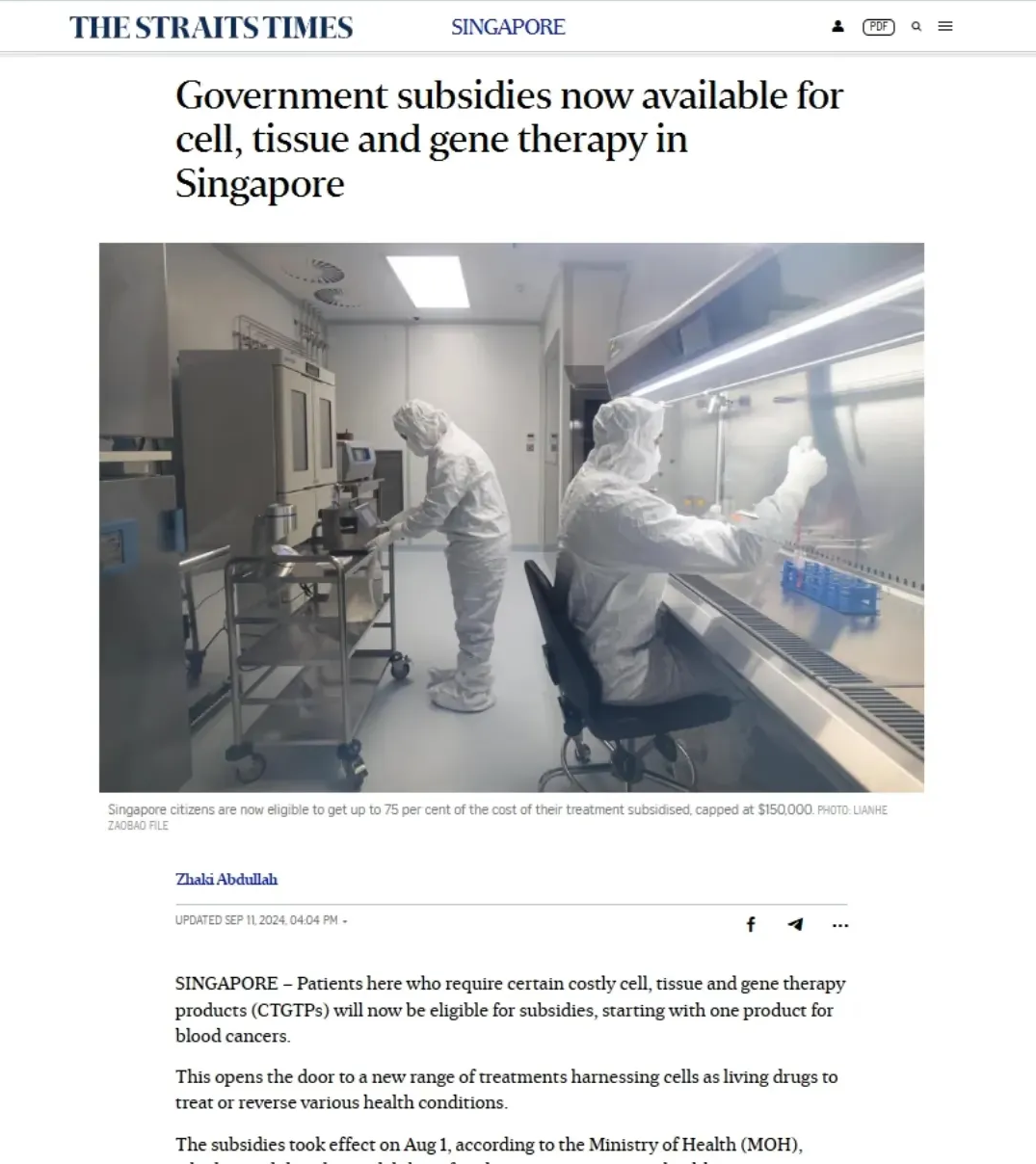
August
Kymriah became the first Cell, Tissue, and Gene Therapy Product (CTGTP) to receive public subsidy under MOH’s pilot financing framework. Means-tested subsidies of up to 75% (capped at $150,000) are now available for eligible patients at public institutions. The CTGTP List reflects the importance of HTA in guiding sustainable funding decisions for high-cost innovations and sets a precedent for future coverage.
Risdiplam, a treatment for spinal muscular atrophy (SMA), was added to the Medication Assistance Fund, allowing eligible patients to receive subsidies of up to 75%. This marked a major step in improving access to life-saving therapies for rare, severely debilitating conditions through evidence-informed policy.
2025 & Beyond
February
Supported the review to extend subsidies and MediSave use for the shingles vaccine—a high-impact but costly preventive treatment—for eligible Singaporeans and Permanent Residents at public healthcare institutions and CHAS-GPs. ACE evaluated its clinical and cost-effectiveness and negotiated value-based prices to improve affordability and access for eligible Singaporeans.
Pneumococcal conjugate vaccine 20-valent (PCV20) was added to the National Adult Immunisation Schedule in 2025, with subsidies for at-risk adults implemented from September.
Collaborating for Impact: Local to Global
Partnering Across Borders and Systems: Learning, Collaborating, Leading
ACE’s role goes beyond national borders, influencing global best practice and bringing international standards home. Through close partnerships across Singapore and abroad, we help shape more consistent, transparent, and value-driven care.
2017

March
ACE established the International Advisory Panel (IAP) to bring in experts advising the agency on global best practices in HTA and clinical guidelines, reinforcing Singapore’s shift towards evidence-based, value-driven care.
2018

August
ACE was appointed Secretariat of the MOH Rare Disease Expert Group (RDEG) expanding its remit to Rare Disease Fund (RDF) evaluation.
2020
February - March
Introduced the inaugural “Health Technology Assessment & the Art of Decision-making” workshop, equipping over 40 healthcare professionals from SingHealth, NHG and NUHS. This initiative helped deepen understanding of HTA’s role in driving value-based healthcare and fostered a community of practice grounded in evidence-based decision-making.
March
Established a panel of academic institutions with HTA expertise to support ACE’s reviews, ensure methodological rigor, independently critique evaluations, and provide evidence for policy decisions.
2021
June
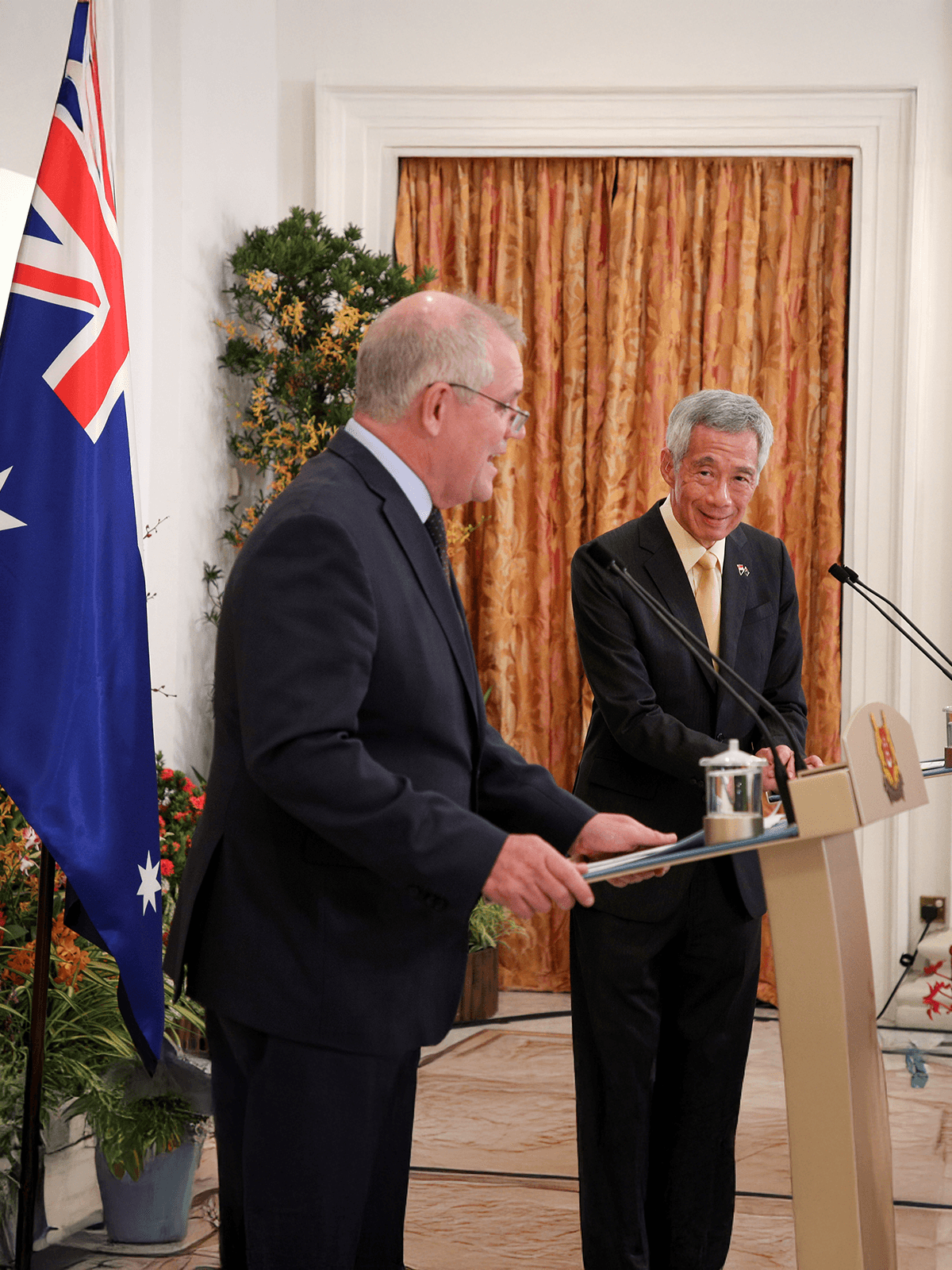
Signed an MOU with the Australian Government Department of Health (AuDOH) to exchange expertise in healthcare and HTA to improve health outcomes for patients.
Former Australian Prime Minister Scott Morrison with former Prime Minister Lee Hsien Loong at the 6th Singapore-Australia Leader's meeting in Singapore, 10 June 2021.
Photo credit: Ministry of Digital Development and Information (MDDI)
2022 & 2024
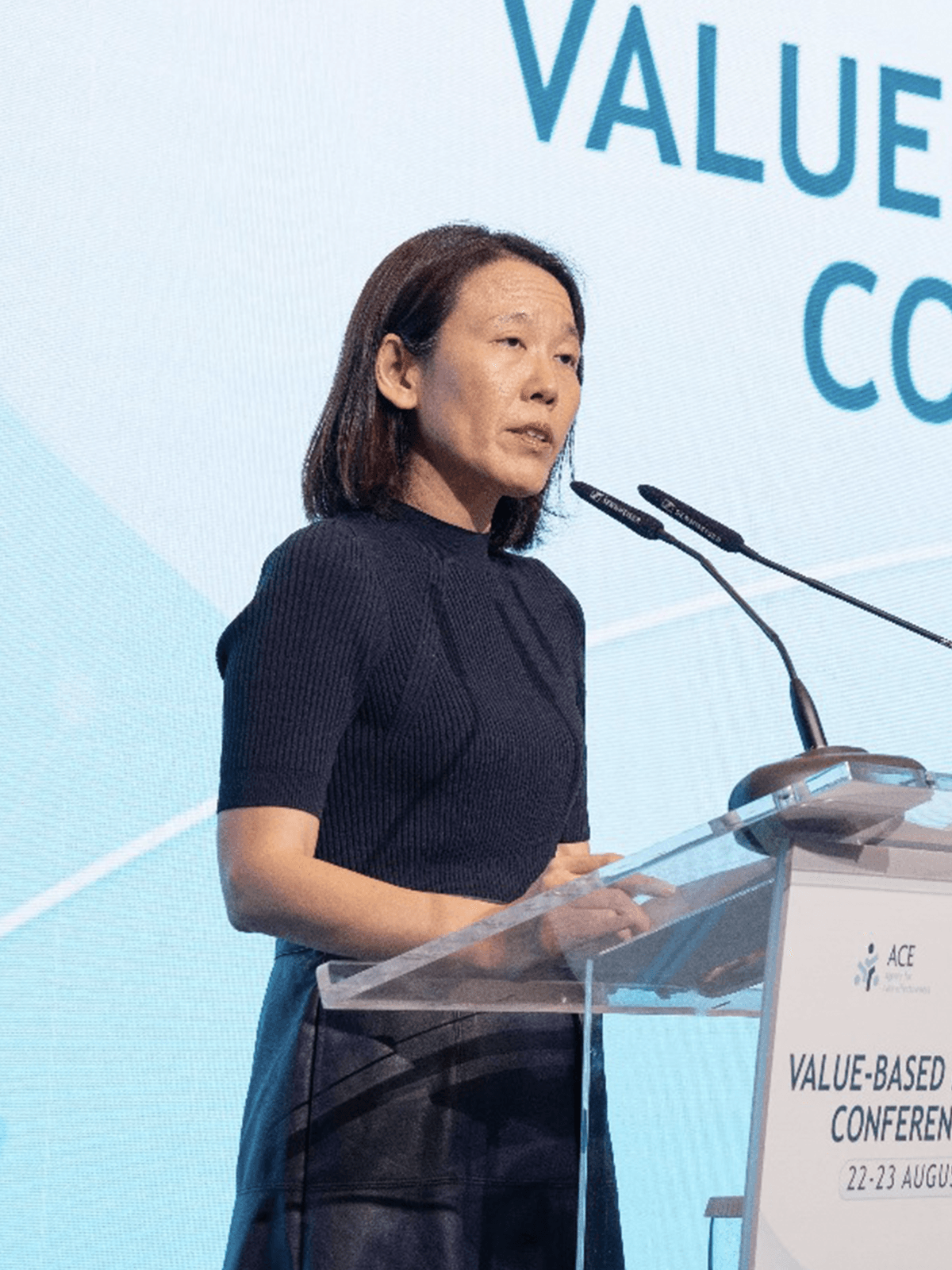
Hosted Singapore’s first national Biannual Value-Based Healthcare (VBHC) Conferences, convening healthcare leaders, policymakers, and experts in value-based care and HTA to share insights and practical strategies. The inaugural conference took place in 2022, with the second edition held at Sands Expo and Convention Centre in 2024.
2025 & Beyond
ACE continues to participate actively in regional and global HTA and guideline networks, including knowledge exchanges through platforms such as HTAsiaLink, HTAi, and GIN, to share best practices and advance value-based healthcare together.
